DEBAMED medical bags: perfect for pneumatic tube transport in university hospitals

In clinics and hospitals, many of the processes connected with internal logistics are hidden to the human eye. But behind the scenes, they keep the “system” up and running. One of the functions of internal logistics is handling the movements of medical samples. These are generally sent back and forth between the main lab, satellite labs and the microbiology department – often via PTT (pneumatic tube transport). PTT is not the mere realm of academic institutions – it is also used by hospitals not affiliated with universities.
RKI requirement: safe packaging for pneumatic tube transport
The Robert Koch Institute (RKI)’s guidelines for hospital hygiene and the prevention of infection list requirements for conveying samples (thus potentially infectious substances) via PTT. These must be packaged in “unbreakable, leakproof, tear-proof packaging”. Before entering the tube system, any containers coming from potentially infectious departments or areas (as defined in clause 5.2.2 in the guidelines) must be disinfected. Before entering or leaving areas which require particular protection against infection (this is defined in clause 5.2.1 of the guidelines), containers must pass through an airlock in the same way as other materials destined for such areas (as per paragraph 7). Hand-washing facilities and hand sanitisers must be provided for the staff who handle incoming or outgoing deliveries.
Sought and found: teaching hospital finds DEBATIN
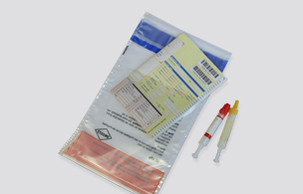
In light of the strict legal requirements, a German teaching hospital found itself in need of a partner to provide secondary packaging for category B medical samples. The challenge lay in finding a hygienic solution for transporting samples internally – by hand or via PTT –that would also meet all requirements for external transportation.
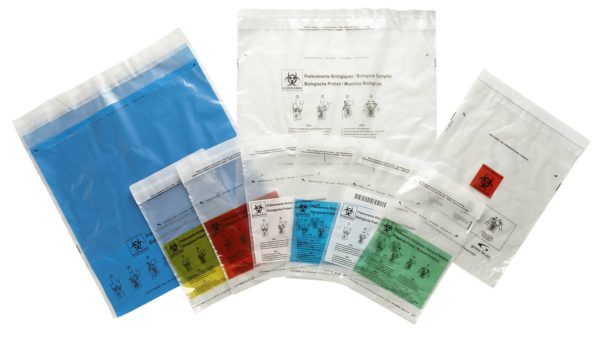
“If samples are being transported internally, regulations such as the ADR (European Agreement concerning the international carriage of dangerous goods by road) don’t apply, of course,” explains Robin Fabry, area sales manager for Anton Debatin GmbH. “However, our customer definitely wanted to protect staff and patients from the dangers posed by contaminated materials.” The answer: DEBAMED® Speci-Bags and DEBASAFE® medical transport bags for samples.
By opting for our leakproof DEBASAFE® medical transport bags, the teaching hospital benefits in a variety of ways:
- The leakproof secondary packaging can be used internally (in line with RKI requirements) as well as externally (in accordance with the ADR). This simplifies the procurement process.
- Thanks to tear notches, no scissors are required to open the mailing bags. This makes life safer and easier for users.
- According to the ADR, packaging must include an absorbent layer. DEBATIN incorporates this directly in the medical bags, meaning the bag is immediately ready for use.
- The mailing bags come in different colours, so they can be assigned quickly and easily to different departments (e.g., microbiology, pathology, virology etc.).
- Thanks to custom printing in up to eight different colours, the mailing bags can be branded with the teaching hospital logo.
Sustainability matters – to us and to our customers!
“The teaching hospital is committed to the UN’s sustainability goals – just like DEBATIN,” says Thomas Rose, CEO of Anton Debatin GmbH. “Our medical bags are made of 85 % PCR materials. These materials, in other words, have already been used by end customers before being professionally recycled. Our medical bags bear the Blue Angel label, certifying that they help protect resources. This fits in perfectly with our customer’s sustainability strategy.”
Mailing bags that withstand aircraft pressure
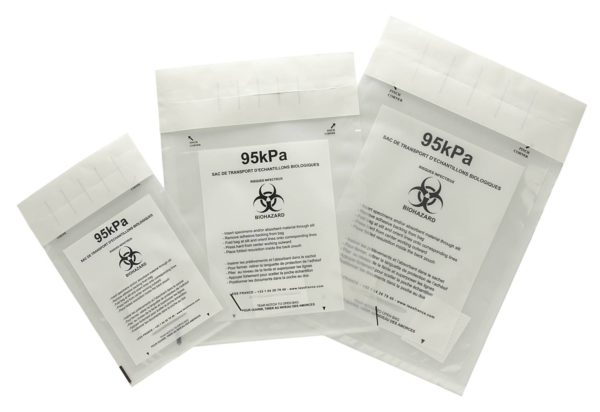
PCR samples, blood samples and the like sometimes need to be transported by road or air. Our DEBAMED® Speci-Bags 95kPa are ideal for such purposes. They’re designed as secondary packaging for transporting category B biological substances (UN 3373) by road or air. They also comply with the international and European standards and guidelines for the transport of infectious substances (ADR – IATA – WHO – ISO 15189). These leakproof sample bags can withstand a pressure difference of 95 kPa / 14 psi, meaning they cope effortlessly with air travel and arrive in perfect condition.
For transportation purposes, dangerous goods legislation distinguishes between the following categories of toxic and infectious substances:
Category A:
This category includes all infectious substances which could permanently disable otherwise healthy humans or animals, or could trigger life-threatening or fatal diseases. For humans, these include the viruses causing Brazilian haemorrhagic fever, smallpox, Lassa fever, Ebola and yellow fever. These are classed as UN 2814 substances.
Category B:
This category includes cultures of infectious substances which do not meet the criteria for category A substances and which are transported for diagnostic or clinical purposes. These substances are labelled as UN 3373. Detailed information is provided in the “Classification of infectious substances” table.
The sender/principal is responsible for classifying the sample correctly and packaging it with the correct declaration. In a hospital setting, this will generally be the physician or the lab manager. Those involved are often unaware of their responsibility in this context. The question may arise as to whether infectious substances can be exempted from regulations during transportation. LQ (limited quantity) exemptions for packaged goods do not apply in such cases. However, certain exemptions from ADR requirements may be made if the following conditions apply:
- If a sample does not contain any infectious substances or substances that are likely to trigger disease in humans or animals (ADR 2.2.62.1.5.1)
- If a sample taken from a human or animal (patient sample) is highly unlikely to contain pathogens, is transported in packaging that reliably prevents any form of leakage, and is labelled as “EXEMPT HUMAN SPECIMEN” or “EXEMPT ANIMAL SPECIMEN” (ADR 2.2.62.1.5.6). In such cases, the substances are not subject to ADR requirements for dangerous goods.